Starting this fall, women will have access to a simple swab test for cervical cancer in a major update to women’s healthcare.
The screening test for cervical cancer, known as a Pap smear, can be so uncomfortable that women avoid going to the doctor because of it.
Around eight million women who should be screened for cancer haven’t been to the doctor in the past five years.
The delay in care translates to more women unknowingly carrying human papillomavirus (HPV), the leading cause of cervical cancer. And the later the diagnosis is made, the more difficult cancer is to treat.
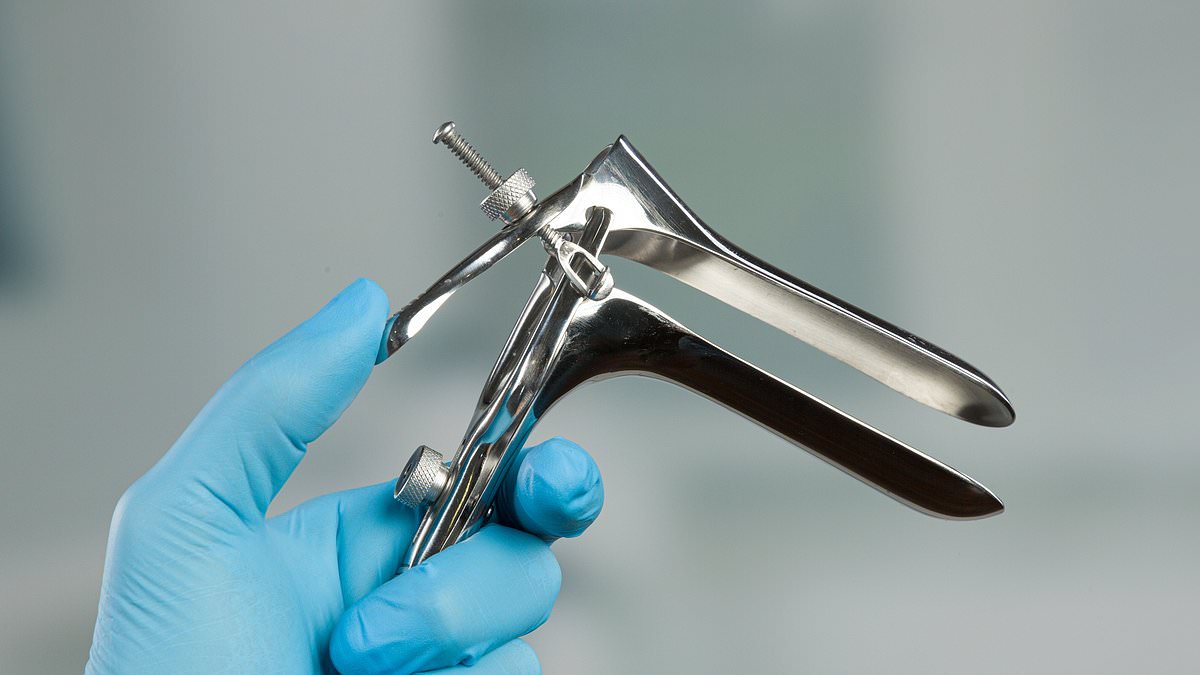
The current in-office procedure involves a device called a speculum, a cold duck bill-shaped metal device that goes into the vagina and widens it to allow the doctor to take a swab of cells on the cervix.
In addition to being physically uncomfortable, it can be emotionally distressing, particularly for people who have experienced sexual abuse.
But a new model of cervical cancer screening has arrived and allows women to circumvent the speculum entirely.

The newly-approved Roche HPV test allows women to self-swab rather than lay down with legs in stirrups. The sample is sent to a lab which screens for HPV, the cause of 95 percent of all cervical cancer cases
In May, the FDA approved two self-collection screening tools for cervical cancer from the pharmaceutical companies Roche and Becton, Dickinson and Company.
The tests are done in a doctors office like a traditional Pap smear, but rather than lying down with legs in stirrups while the doctor performs the uncomfortable procedure, the new tests allow women to go into a room, swab their vaginal canals themselves, and leave a sample with the doctor, similar to leaving a urine sample.
In traditional Pap smears, the doctor swabs the cervix and deposits the sample onto a slide and sends it to a cytopathologist, a lab professional who is especially trained to examine cells under the microscope.
The primary goal of a Pap smear is to identify abnormal cells in the cervix that may indicate cervical cancer or precancerous conditions.
The newly-approved self-collections are sent to labs that are now able to test samples from vaginal walls as opposed to the cervix.
The lab tests the sample for strains of HPV most likely to cause cervical cancer.
Dr Karen E. Knudsen, CEO of the American Cancer Society, said: ‘Almost all cervical cancers are caused by persistent infection with certain types of HPV.
‘Self-collection can expand access to screening and reduces barriers, which will give more people the opportunity to detect, treat, and ultimately survive cancer.’
The lab relays those results to the doctor who ordered the testing, and the doctor meets with their patient to discuss them.
By detecting possible HPV, the doctor is then able to determine whether the patient is at risk of developing cervical cancer by testing for precancerouschanc
Dr Shieva Ghofrany, an OB-GYN and Fellow of the American Congress of Obstetricians and Gynecologists, said: ‘The integration of self-collection with testing for individualized strains of HPV represents a significant advancement in cervical cancer screening.
‘Self-collection provides greater access to testing and [the Becton, Dickinson and Company test] allows health care providers to determine the specific HPV strains present in the samples and more precisely identify and treat individuals at high-risk and avoid unnecessary treatments for women at low risk.’

The speculum is a cold duck bill-shaped metal device that goes into the vagina and widens it to allow the doctor to take a swab of cells on the cervix
But a growing wave of women are railing against the deeply entrenched notion that women’s healthcare involves pain and must be accepted.
By detecting possible HPV, the doctor is then able to determine whether the patient is at risk of developing cervical cancer by testing for precancerous changes in cervical cells.
Researchers are hoping to take their innovation a step further and introduce at-home testing that would negate the need for an potentially awkward and painful doctor’s appointment.
It would work in the same way that colorectal cancer screening kits do: a woman would take a swab of her vaginal canal, packages it, and mails it to a lab, which tests for HPV. If the test returns a positive result, the patient and her doctor can discuss steps
The Pap smear has gone relatively unchanged since its inception in the 1940s. It was a revolutionary medical development that has helped slash cervical cancer rates by 70 percent since the pre-screening days.
But a growing wave of women are railing against the deeply entrenched notion that women’s healthcare involves pain and must be accepted.
The dreaded IUD, a notoriously painful form of contraception, is in the midst of a makeover.
Experts at Swiss tech company Aspivixhave invented an alternative method that uses suction to manipulate the cervix, slashing pain in a study by three-quarters.
Menopause symptoms are also getting overdue attention, with several menopause-focused drugs in the pipeline.
The FDA approved the first-ever drug last year specifically to treat hot flashes caused by menopause. Roughly 80 percent of menopausal women get hot flashes.