The Jesuits have a saying for how to win a recruit for life: ‘Give me a child until he is seven and I will show you the adult.’
Now it seems drug companies may be taking the same tack – by suggesting young children could benefit from drugs that they may then need for decades if not for ever.
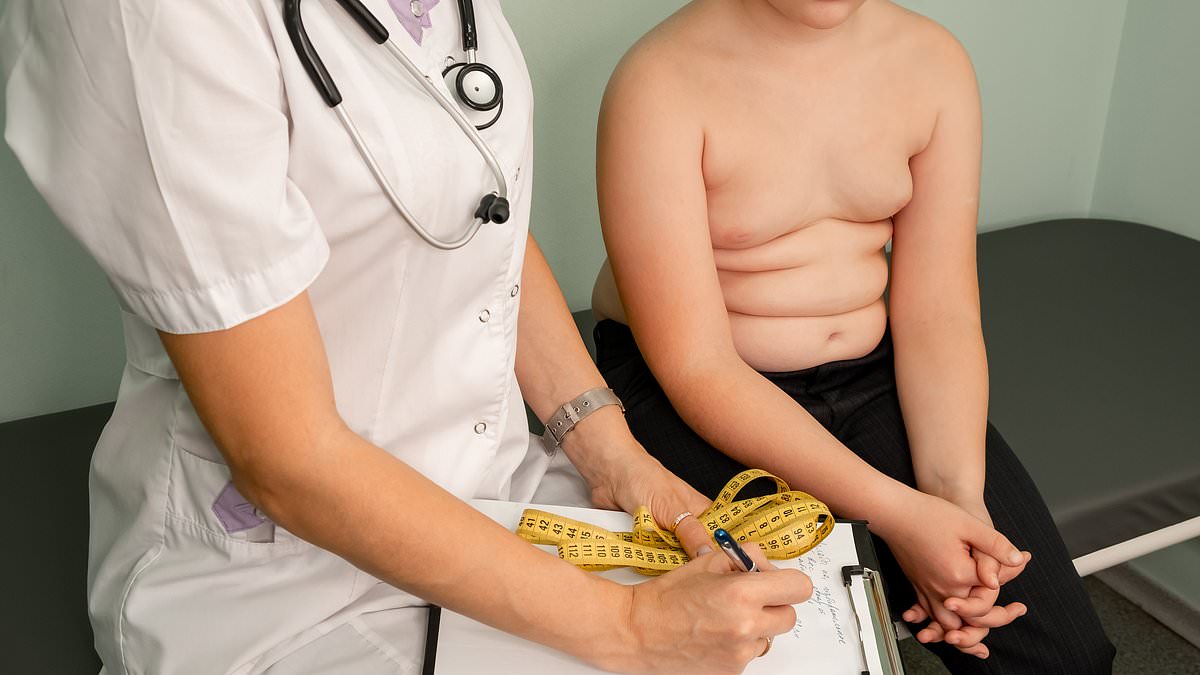
Good Health has discovered that the makers of the fat jab Wegovy are lobbying for this type of medication to be given to overweight children as young as six.
This follows revelations last month made in a Channel 4 Dispatches TV documentary that a girl of 16 was able to pick up Wegovy from Boots despite the fact that it’s meant to be available only to those aged 18 and over.
However, Good Health has also discovered that NHS trusts are already giving weight-loss jabs such as Wegovy to children as young as ten as a treatment for obesity – even though they have not been approved for this age group by the National Institute for Health and Care Excellence (NICE), a health watchdog.
What is undeniable is that there is a large potential market for the drugs, known as GLP-1 agonists, as they mimic the effects of the hormone GLP-1 which slows the digestion of food and reduces appetite.
British and German children are the joint fattest in Europe, according to research published in The Lancet last month. According to statistics published by the House of Commons in February, more than a quarter (26.8 per cent) of children in England aged two to 15 are now overweight or obese.
The appeal of having something like Wegovy to offer is obvious – a teenager in the US who was put on Wegovy by an obesity medicine specialist described how she lost over 3 stone (50lbs) after previous attempts over the years using diet and exercise had failed. The girl, named only as Ann A, said she had even tried a summer weight-loss camp but soon after, she put the weight back on and ended up heavier than she’d been before she went in. Meanwhile, as her weight rose, she became pre-diabetic and her levels of blood fats became abnormally high.

More than a quarter of British children are considered to be overweight or obese, with the country in joint first place with Germany for having Europe’s fattest kids
Now she says: ‘I feel better not just physically but mentally.’
Ann is 18 but Wegovy’s Danish manufacturer, Novo Nordisk, is applying to European and American regulators to have a GLP-1 jab approved for children as young as six.
And, as we shall hear, some experts are deeply concerned this will have major implications for their future health.
Novo Nordisk started laying the groundwork for giving children GLP-1 drugs in September with the publication of a study it funded, in the highly reputed New England Journal of Medicine.
This compared two groups of children aged six to 11. Fifty-six were given the GLP-1 drug liraglutide (which is available under the brand name Saxenda, made by Novo Nordisk) and 26 received a placebo. Both groups also received lifestyle advice on diet and exercise.
After 18 months, the study concluded that the weight-loss jab liraglutide was much more effective than the placebo (the youngsters had a 5.8 reduction in their BMI score with liraglutide versus 1.6 per cent with the placebo).
Those on the drug had the option to stay on it afterwards. More than one in ten of the children given liraglutide, however, stopped taking it even before the trial ended, due to unbearable side-effects, mainly gastrointestinal problems, including stomach upsets, vomiting and nausea (as have been seen with adults taking GLP-1 drugs).
And experts fear that children who stop taking GLP-1 drugs will see their weight rebound – the study noted that when treatment was stopped, BMI started to rise back up.

Last month a Channel 4 Dispatches documentary found that a girl of 16 was able to pick up Wegovy from Boots despite the fact it’s meant to be available only to those aged 18 and over
In an editorial in the New England Journal of Medicine accompanying the Novo Nordisk-funded study, researchers from the universities of Birmingham and Bristol said that weight increases among trial participants after liraglutide treatment stopped were ‘worrisome’.
Yet this shouldn’t come as a great surprise given that this is what often happens with adults.
A University of Liverpool study involving 1,900 overweight adults who took the GLP-1 drug semaglutide for 17 months and then stopped, found that 13 months later, the participants had regained on average two-thirds of the weight they’d lost, reported the journal Diabetes, Obesity & Metabolism in 2022.
Thus the prospect of children staying on these drugs long term seems increasingly possible.
However, no one knows the long-term effects, as children were not included in the original trials that led to the approval of the drugs.
Claudia Fox, a professor of paediatric gastroenterology at the University of Minnesota, and who led the New England Journal of Medicine study, said at the time the paper was published: ‘With such a small sample [of 56 children], it’s hard to know if there are more rare side-effects that could have emerged or will emerge. We need much longer-term data and much bigger numbers.’
Despite such question marks, a spokesman for Novo Nordisk told Good Health that it has submitted an application to both the European Medicines Agency and the US Food and Drug Administration to expand the approval of liraglutide for weight loss in children aged six to 11.
The spokesman said the application was based on data from the New England Journal of Medicine trial.
In the UK, there is no official regulatory approval for giving liraglutide to treat obesity in anyone under 18. In 2021, NICE said that Novo Nordisk had not provided it with any evidence to show the drug’s effectiveness in children or adolescents.
But across the NHS, hospital trusts are already treating children as young as ten with liraglutide.
Following a freedom of information request by Good Health, the University Hospitals of Leicester NHS Trust revealed that it has given the jabs to 20 children aged between 12 and 18 in its paediatric obesity service, and has been using the medication in this age group since 2021 (the trust says it has also treated ‘two or three patients in the Paediatric Diabetes Service’ with liraglutide, though somewhat confusingly NICE says it does authorise the drug’s use for children ‘aged 10 or over with type 2 diabetes’).
Meanwhile, the NHS Hampshire and Isle of Wight Integrated Care Board’s prescribing guidelines recommend giving GLP-1 drugs to severely obese, non-diabetic children as young as 10.
A spokeswoman for the authority told Good Health: ‘Prescribing of Wegovy and Ozempic for children is only supported in exceptional circumstances via our specialist paediatric weight-management services, carried out at specialist centres in Portsmouth Hospitals University Trust and University Hospital Southampton.
‘Only children struggling with severe obesity that haven’t been able to be controlled through support with eating and exercise are referred to our specialist paediatric weight-management service.’ The spokeswoman would not reveal how many children the hospitals had treated.
In a similar vein, a spokeswoman for the University Hospital Southampton NHS Foundation Trust would only confirm ‘that this is a treatment we provide to a very small number of young patients under the age of 18’, but declined to reveal the numbers of children being treated ‘due to the sensitive nature of this treatment and the vulnerability of those involved’.
Dr Nikki Davis, a consultant in paediatric endocrinology and diabetes at the trust, added: ‘GLP-1 medications are used very rarely in children alongside holistic weight-management programmes and only when all other options have been exhausted.’
Other NHS local authorities take a very different tack. NHS Lancashire and South Cumbria Integrated Care Board, for example, has a ‘do not prescribe’ marker on its instructions for medical staff (but openly visible to the public online) on ‘Liraglutide for managing obesity in people aged 12 to 17 years’, citing the NICE guidance.
Even NHS England does not know how many children are being prescribed GLP-1 drugs and under what circumstances – even when these drugs are given under the heavily restricted NICE approval for type 2 diabetes only.
The latest NHS National Diabetes Audit, published 15 months ago, admits that prescription figures for GLP-1 drugs given to children were not included in the data collection. The report says: ‘This means that individuals may have been omitted when they should have been included.’ Yet the NHS appears to be unconcerned, despite the lack of information.
A spokesman for NHS England told Good Health: ‘These treatments are strictly prescribed under specialist paediatric supervision, following careful assessment and in line with national guidance – ensuring they are safe, clinically appropriate and combined with a comprehensive care package including expert dietary, physical activity and mental wellbeing support.’
The Royal College of Paediatrics and Child Health would not comment when contacted by Good Health about prescribing GLP-1 drugs to children as young as six. But across the Atlantic, experts are sounding alarms.
Dan Cooper, a professor of paediatrics at the University of California, Irvine, told Good Health that he was ‘very worried’ that these drugs may harm children’s developing bones and brains – and ultimately may leave them severely frail and living with seriously damaged mental health. Two years ago, Professor Cooper led a team of clinicians, exercise scientists, pharmaceutical scholars, ethicists and behavioural experts in warning of the potentially disastrous consequences that mass-medicating youngsters with GLP-1 drugs could cause.
That warning was published in the journal Clinical and Translational Science – but the professor says the world is not listening.
He is particularly concerned that children who are started on GLP-1 may be beginning a life sentence. He asks: ‘Are we going to tell kids on GLP-1 drugs that they have such a fundamental flaw they cannot manage to learn the habits of having healthy physical activity and a healthy diet?
‘These medications are remarkable for their effectiveness. But they are not a drug that fixes you and then you stop taking them. You just have to keep taking them, perhaps for life. How will that affect a child? The idea of giving GLP-1s to six-year-olds makes me very nervous.’
Professor Cooper is particularly concerned about the drugs’ impact on youngsters’ development.
‘Adolescence is a critical period of growth,’ he warns. ‘We don’t know enough about GLP-1 drugs’ long-term effects on this age group to say how it will affect them.
‘We don’t know how it will affect their development through puberty, which biologically is a hugely complex stage.
‘But we do know that we are giving them a medication that creates an imbalance in their energy intake, and this will affect bone mineralisation [bone strength] – and beyond adolescence you can’t add bone.
‘The same problems will arise with children’s development of vital muscle.’
Furthermore, he says, he is ‘particularly worried about the effect on their mental health’.
‘Before we recommend widespread use of these drugs in children and adolescents, we need to understand the consequences on their developing brains,’ Professor Cooper says. ‘This will disrupt them in very profound ways.
‘The fact is that GLP-1 receptors are found in the brain as well as in the body, and I believe these drugs are affecting how children think.
‘Already you see in kids on these medications that they don’t want to get out of bed in the morning. Their youthful zest is gone.’
It would be a terrible irony if a drug for children’s fast-increasing obesity problems actually worsened this age group’s mental health problems, at a time when young people are increasingly being put on psychiatric drugs that they may also end up taking for life.
Because this isn’t a problem limited to obesity medication.
Last year, a panel of British experts led by David Branford, an associate professor of pharmacy at Plymouth University, warned in the British Medical Journal’s Drug and Therapeutics Bulletin that the prescribing rate of antipsychotics for children had risen by more than 3 per cent every year between 2000 and 2019.
At the same time, the prescribing rate of antidepressants more than doubled among 12 to 17-year-olds between 2005 and 2017, the report said.
The panel warned that doctors were prescribing psychiatric drugs to young patients for ever longer periods despite ‘serious concerns about these psychiatric drugs’ safety and efficacy in children and young people’.
As Professor Cooper at the University of California, Irvine, puts it: ‘I’m very concerned at how the world is increasingly medicating everything.
‘Are we really prepared to have children growing up believing that the only way that they can survive is to be on medication for the rest of their lives?’